As part of the Rotman Institute Speaker Series, each year one speaker is chosen as the Rotman Institute Lecturer in Philosophy and Science. This year, the Rotman Institute had the pleasure of hearing from Professor John D. Norton as the 2012/2013 Rotman Lecturer. Norton is currently the Director for the Center for Philosophy of Science, and Professor in the Department of History and Philosophy of Science at the University of Pittsburgh. Norton is perhaps best known for his work in the philosophy of physics, and as a world expert on Albert Einstein’s philosophical approach to physics.
The Rotman Lecturer is asked to deliver two talks; one aimed at the general public, and the other as a more detailed lecture on their current research. On March 14th Norton delivered his public lecture on “Einstein as the Greatest of the 19th Century Physicists”, and on March 15th Norton spoke on his recent paper, “Approximations and Idealizations”.
Ideally, the Rotman Institute should host John Norton at least approximately once a year
John Norton’s second lecture was on the difference between approximation and idealization. This distinction was the object of a paper, published in Philosophy of Science. It contributes to ongoing philosophical discussions (involving among others Jeremy Butterfield) around Robert Batterman’s work. These discussions concern broad topics such as the nature of scientific reasoning, explanation, emergent properties, and intertheoretic reduction. This debate is especially relevant to the relation between statistical mechanics and thermodynamics, and the physics of phase transition.
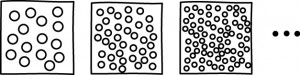
Norton distinguished approximations (inexact descriptions of real systems) from idealizations (exact descriptions of idealized systems, akin to models). To these two sorts of analytic activities correspond two ways to take the infinite limit: whereas approximations consider the limiting properties of finite systems, idealizations may involve infinite systems (system with infinitely many components). But infinite systems can have unexpected or inadequate properties (for instance, they may violate determinism and energy conservation). Therefore, idealization is a riskier activity. Thus, when seen as an idealization and not an approximation, the thermodynamic limit must suffer a higher degree of scrutiny.
This distinction also allowed Norton to address the description of renormalization groups and claim that, contrary to what is sometimes argued, they are approximations, not idealizations. As a reply to Kadanoff, Norton then asserted that an infinite system is not necessary to phase transitions, for if it were, it would refute the atomic theory.
Norton then turned to the issue of whether phase transitions are an example of reduction (of thermodynamics to statistical mechanics) or, on the contrary, of emergence (of thermodynamic properties). In an additional note, Norton claimed that both sides of the debate are right. In that, he agrees with Jeremy Butterfield. But Norton brought clarity to why it is so—and that was the particular addition of this talk to the paper. Confusions arise when the nature of the levels (reduced or emergent) isn’t clear. There is reduction between theory-levels (from the thermodynamic to the molecular-statistical level), but there is emergence between scale-levels (from a scale of a few molecules to one of many components). Norton conjectured that the confusion may stem from differences in disciplinary practices: whereas philosophers tend to divide levels by theory, physicists tend to do it by scale.
Of course, Norton’s arguments are relevant beyond the issue of phase transition. They provide us with distinctions that are necessary to identify the domain of validity of explanatory models and how they relate to one another. Norton’s work clarifies why explanations, if they involve idealizations, are limited in scope.
(Many thanks to Melissa Jacquart for her help.)